Clam
Dissection
Clam
Dissection
 Clam
Dissection
Clam
Dissection
If you were absent and missed the lab, click HERE for a video of the clam dissection.
Background Information:
The phylum Mollusca includes snails,
clams, chitons, slugs, limpets, octopi, and squid. As mollusks develop from
a fertilized egg to an adult, some mollusks pass through a larval stage called a
trochophore. The trochophore is a ciliated,
free-swimming stage (see diagrams below). Some mollusk larvae develop from a
parasitic larval form known as a glochidia.
(see page 8 of the dissection guide to learn more about the glochidia).
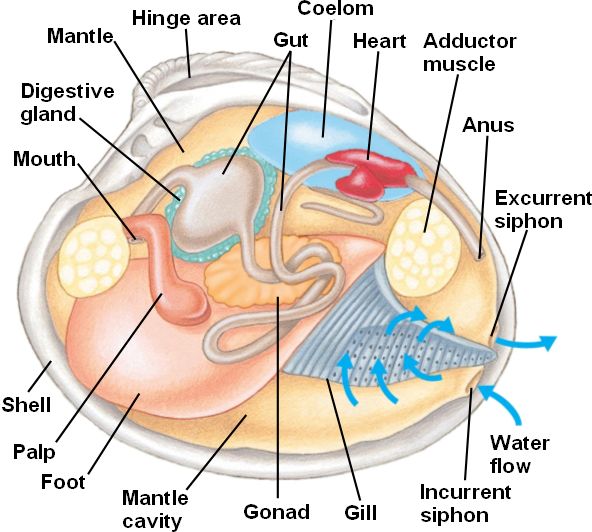
Mollusks also have a radula or file-like organ for feeding, a mantle that may secrete a shell, and a muscular foot for locomotion. Clams are marine mollusks with two valves or shells. Like all mollusks, a clam has a mantle which surrounds its soft body. It also has a muscular foot which enables the clam to burrow itself in mud or sand. The soft tissue above the foot is called the visceral mass and contains the clam's body organs.
Mollusk Trochophores


Mollusk Glochidia
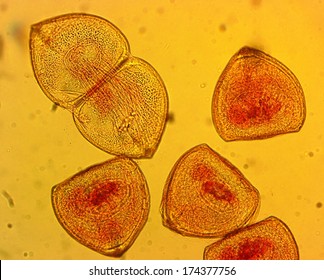

Here you can see the glochidia within the gill system of a fish.
Taxonomy of the Clam (the species you are dissecting)
| Kingdom | Animalia |
| Phylum | Mollusca |
| Class | Bivalvia or Pelecypoda |
| Order | Unionida |
| Family | Unionidae |
| Genus | Anadonta |
| Species | californiensis |
Therefore the scientific name of the clam you are dissecting is: Anadonta californiensis. The common name of this river mussel is the "California floater". The range of the California floater may include Idaho, California, Utah, Washington, Arizona, Wyoming, Nevada, and Mexico. The shells of the California floater are thin and do not close completely which results in a clam that is easier to open for dissection thus more inexpensive to process and ship. This makes them an ideal specimen for classroom dissections.
Morphology
External
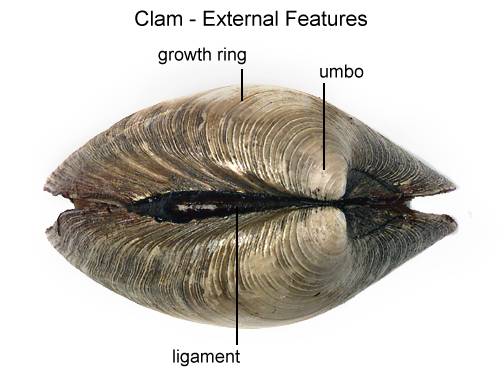
Freshwater bivalves, as their name implies, are composed of two halves, or a left and right valve, connected via a soft ligament along a hinge. These two valves are non-living, composed of both organic and inorganic substances that make up three major valve layers. The first, outermost layer is the thin epidermis (periostracum), followed by a second prismatic layer, containing calcium carbonate. The third and innermost layer is also the thickest and is most commonly referred to as the mother of pearl--- a widely harvested source for the production of ornamental buttons. The external appearances of these shells can be extremely variable when comparing members of different families, genus, etc., as well as intraspecies. Valve surface appearances can range from smooth to dramatically sculpted, showcasing ornamental pustules, pimples, grooves, and ridges. The overall shape of the valve can also vary drastically, from laterally compressed and narrow, to wide and globular.
Internal
The mantle is a multifunctional, generally thin and fragile structure that line bivalve interiors and encloses their bodies. This structure secretes the shell, contains respiratory organs (facilitates respiration), and facilitates feeding. The cavity that exists between the mantle and other soft tissues is aptly named the mantle cavity.
Within the mantle cavity on either side of the foot are the gills. The inner gills are adjacent to the foot and the outer gills nearest to the mantle and shell. As expected, these gills mainly act as a respiratory structure, facilitating gas exchange but can also facilitate in feeding. Water can enter the mantle cavity via an incurrent siphon, and pass over the gills where food and other particles are trapped by secreted mucus.
Reproduction
Freshwater Bivalves can utilize either ovoviviparous or viviparous reproduction strategies, ovoviviparous meaning that embryos develop and grow in eggs inside the female until they are ready to be released. The embryos get nutrition from egg yolk, but are not connected to the mother by a placental connection. Viviparous meaning embryos develop inside the body of a female and usually gain nutrition by a placental connection. Females tend to have a single reproductive spawning period (when the ova moves to the gills) while males tend to have two spawning periods (a release of sperm into the water column). Females have highest ova, or mature female reproductive cells, during September with a gradual decrease until December. Males spawn between September and December with a second spawning period between May and July. Males release their sperm into the water column for females to accept. Females take up the sperm along with water through their circulatory system and have the potential to become fertilized when the sperm meets the ova. In contrast to Marine Bivalves, most female freshwater bivalve species hold the fertilized embryos until they develop into larvae when they are released into the water.
Larvae
Once larvae are released into the water column they become semi-parasitic and attach to the gills of freshwater fish. They attach to a host where they grow into juvenile adults while doing little to no damage to the fish host. The order Unionidae have an obligate parasitic larval stage where the larvae are attached to the gills, fins or the body of a particular host fish. Microbial water composition and sediment composition are important in larval nutrition.
Predation
Invasive species pose a risk to freshwater bivalve populations. Specifically two invasive species of crayfish, Procambarus clarkii and Pacifastacus leniusculus, are predators of the freshwater bivalve species Anodonta anatina. In general, freshwater bivalves have predators such as raccoons, otters, some species of fish, and some species of turtles. Droughts, floods, and heat waves are a few examples of major climatic events that are happening more frequently because of the global changing climate. This is a huge killer of freshwater bivalve populations.
Ecosystem Function
Freshwater bivalves are important bioindicators of freshwater ecosystems because they are the connection between the water column and the benthos, for they can provide information on the quality of the water based on the particles and toxins that bioaccumulate in the tissue of bivalves. Freshwater bivalves are filter feeders and provide an ecological service by improving water quality in the bodies of water they inhabit, such as rivers, lakes, and wetlands. Water quality is improved by filtering out fine particles of silt, organic matter, and heavy metals as well as bacteria and phytoplankton in the water column to reduce turbidity.
Freshwater bivalves are also important in the process of
nutrient cycling because they deposit organic matter in the sediment through
biodeposition created from the fine particles they filter in. Organic matter can
be deposited in the sediment as feces or dead matter, and depending on if the
right environmental conditions are present, such as hypoxia, sediment
denitrification can occur, releasing nitrogen back into the atmosphere. However,
other organisms are unable to utilize this inorganic form of nitrogen, so
freshwater bivalves can also excrete dissolved nutrients in an accessible form
for primary producers and consumers to assimilate. Any nutrients that were
retained by the freshwater bivalve through its lifespan for building shell
tissue can serve as a long-term nutrient storage in the benthos when the
organism dies, depending on water chemistry and flow conditions. Considering
freshwater bivalves can filter particles and process nutrients in the nutrient
cycle, there are other species of freshwater bivalves that have more specialized
ecosystem functions as well as different vulnerabilities.
Threats to Bivalves
Invasive Species
Example of Dreissena polymorpha (Zebra Mussel).
Dreissenidae are a family of freshwater mollusks considered
to be an invasive species found across Eurasia and North America. The most
common types of dreissenids are Dreissena polymorpha (Zebra mussel) and
Dreissena rostriformis (Quagga mussel). These mussels damage both ecological
systems and human infrastructure. In North America, bio-fouling caused by
dreissenids created 267 million dollars’ worth of damage between 1989 and 2004.
When introduced to freshwater ecosystems, dreissenids lead to a decline in
indigenous marine animal populations and are also known for causing benthic
algae and cyanobacterial blooms. The total impact of dreissenids on freshwater
ecosystems is still unknown.
Anthropogenic Impacts
Pollution, human disturbance, invasive species, and ecosystem modification are the main threats to freshwater bivalves. In North America freshwater bivalves are extremely threatened, with 202 of 300 species considered critical, possibly extinct, or extinct. Of the dangers facing freshwater bivalves, eighty-five percent of them are considered to be “ongoing threats”. Ecosystem modification and pollution are currently the two biggest threats to molluscs and gastropods in Palearctic and Nearctic ecozones. Pollution is the dominant issue for these animals in the Afrotropic and Indomalayan biogeographic realms. For the Neotropics and Australasia biogeographic realms, ecosystem modification has the largest impact on freshwater bivalve species. Hydropower plants and dams are two examples of human ecosystem modification which contributes to loss of habitat as well as changes to channel morphology, river and floodplain connectivity and nutrient limitation. Rates of extinction among freshwater bivalves are higher than those of terrestrial groups which share the same ecosystem. Among those bivalves, freshwater gastropods are the most highly threatened due to smaller species distribution. Freshwater bivalves are at a heightened risk for endangerment and extinction because of the connectivity of river systems. Anthropogenic impacts on rivers spread throughout the whole ecosystem.
Objective:
To study the internal and external
anatomy of a bivalve mollusk.
Procedure: Click HERE to access a dissection manual for the clam.
The PDF manual format must be rotated and reduced to about 33% to view it properly.
Place a clam in a dissecting tray and identify the anterior and posterior ends of the clam as well as the dorsal, ventral, & lateral surfaces. (See Figure 1)
Figure 1

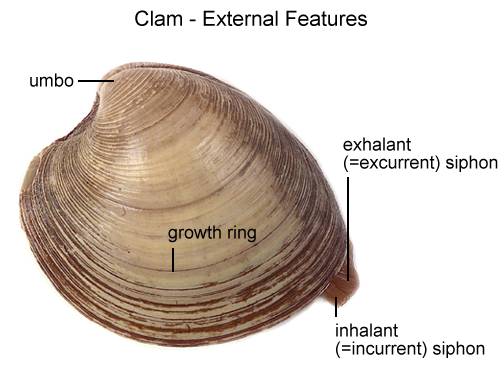
Locate the umbo, the bump at the anterior end of the valve. This is the oldest part of the clam shell. Find the hinge ligament which hinges the valves together and observe the growth rings. The growth rings will indicate the relative age of the clam. Not so much in years, but each additional ring indicates another season of growth. The clam will also use the valves as a way to move quickly. By opening and closing these valves, it can escape predators. It will also use its muscular foot for movement as well.
Turn the calm with its dorsal side down and insert a closed scissors between the ventral edges of the valves. Carefully work the tip of the closed scissors between the valves so you do not jab your hand.
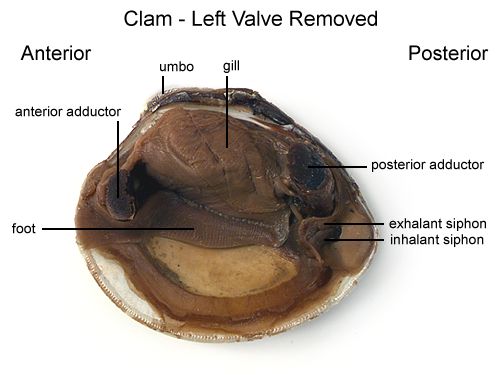
Locate the adductor muscles. With your blade pointing toward the dorsal edge, slide your scalpel between the upper valve & the top tissue layer. Cut down through the anterior adductor muscle, cutting as close to the shell as possible.

Repeat the procedure above to cut the posterior adductor muscle. Figure 2
Figure 2
Bend the left valve back so it lies flat in the tray.
Run your fingers along the outside and the inside of the left valve and compare the texture of the two surfaces.

Examine the inner dorsal edges of both valves near the umbo and locate the tooth-like projections. Close the valves & notice how the tooth-like projections interlock.
Locate the muscle "scars" on the inner surface of the left valve. The adductor muscles were attached here to hold the clam closed.
Identify the mantle, the tissue that lines both valves & covers the soft body of the clam. Find the mantle cavity, the space inside the mantle.
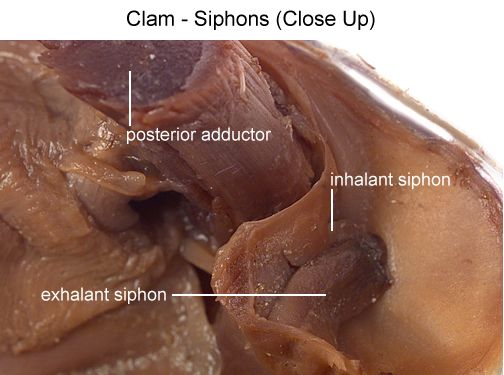
Locate two openings on the posterior end of the clam. The more ventral opening is the incurrent siphon that carries water into the clam and the more dorsal opening is the excurrent siphon where wastes & water leave. Clams will use the siphons to filter out materials in the water for food.
With scissors, carefully cut away the half of the mantle that lined the left valve. After removing this part of the mantle, you can see the gills, respiratory structures.
Observe the muscular foot of the clam, which is ventral to the gills. Note the hatchet shape of the foot which is used to burrow into mud or sand.

Locate the palps, flap-like structures that surround & guide food into the clam's mouth. The palps are anterior to the gills & ventral to the anterior adductor muscle. Beneath the palps, find the mouth.
Figure 3

With scissors, cut off the ventral portion of the foot. Use the scalpel to carefully cut the muscle at the top of the foot into right and left halves.
Carefully peel away the muscle layer to view the internal organs.
Locate the spongy, yellowish reproductive organs.
Ventral to the umbo, find the digestive gland, a greenish structure that surrounds the stomach.
Locate the long, coiled intestine extending from the stomach.
Follow the intestine through the calm. Find the area near the dorsal surface that the intestine passes through called the pericardial area. Find the clam's heart in this area.
Continue following the intestine toward the posterior end of the clam. Find the anus just behind the posterior adductor muscle.
Use your probe to trace the path of food & wastes from the incurrent siphon through the clam to the excurrent siphon.
Click HERE for the Clam Dissection Lab Companion. (Identifies what you will need to know about the clam for the lab exam.)